
Silver is found in native form, as an alloy with gold ( electrum), and in ores containing sulfur, arsenic, antimony or chlorine.
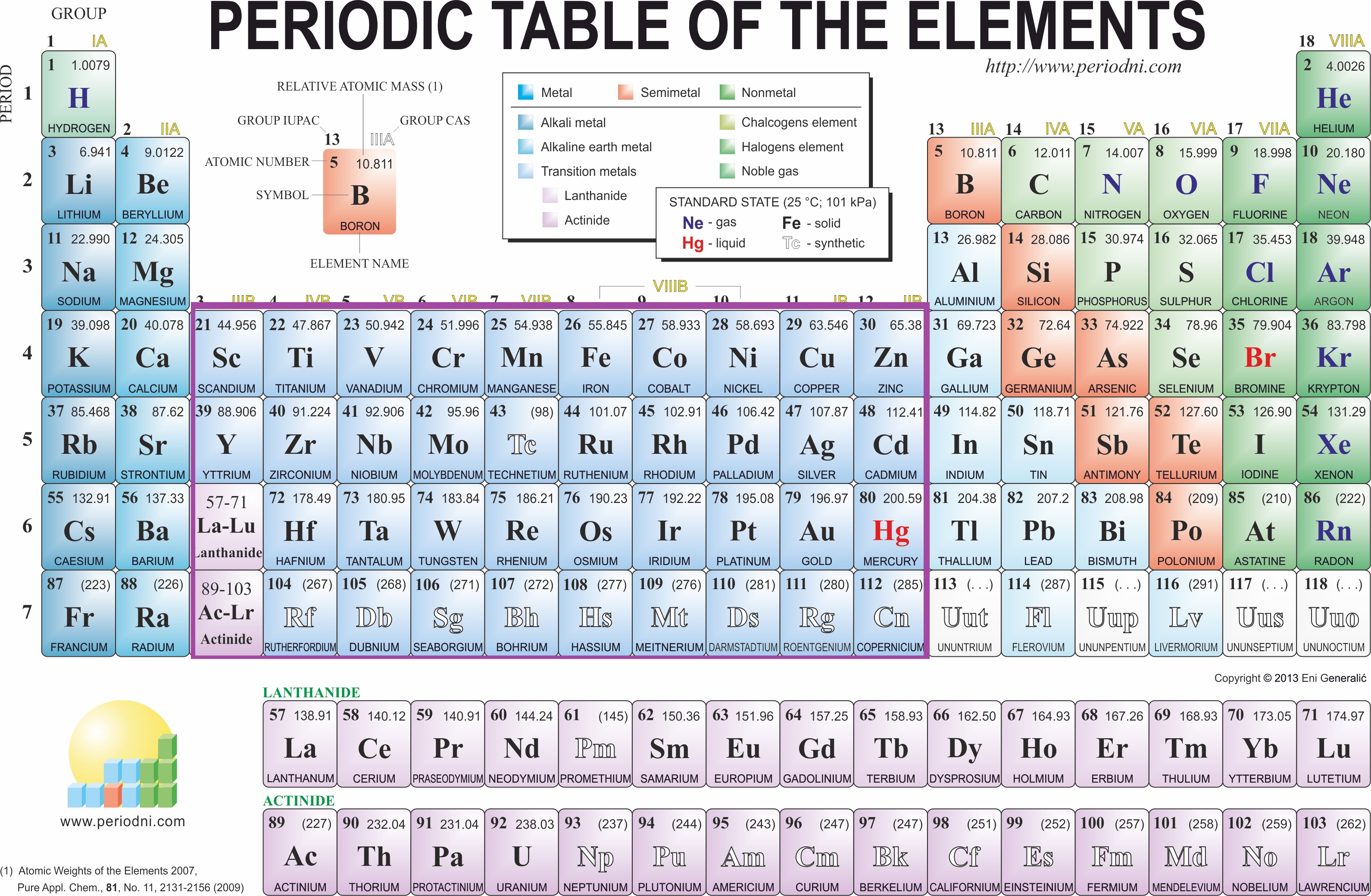
Various natural ores of copper are: copper pyrites (CuFeS 2), cuprite or ruby copper (Cu 2O), copper glance (Cu 2S), malachite, (Cu(OH) 2CuCO 3), and azurite (Cu(OH) 22CuCO 3).Ĭopper pyrite is the principal ore, and yields nearly 76% of the world production of copper. Silver and silver-plated copper wiring are found in some special applications.Ĭopper occurs in its native form in Chile, China, Mexico, Russia and the USA. Bond wires for integrated circuits are usually gold. Copper is the cheapest and most widely used. These elements have low electrical resistivity so they are used for wiring. Roentgenium is expected to be silvery, though it has not been produced in large enough amounts to confirm this. Copper and gold are colored, but silver is not. Like other groups, the members of this family show patterns in electron configuration, especially in the outermost shells, resulting in trends in chemical behavior, although roentgenium is probably an exception:Īll group 11 elements are relatively inert, corrosion-resistant metals. Roentgenium was made in 1994 by bombarding nickel-64 atoms into bismuth-209 to make roentgenium-272. Gold artifacts made their first appearance at the very beginning of the pre-dynastic period in Egypt, at the end of the fifth millennium BC and the start of the fourth, and smelting was developed during the course of the 4th millennium gold artifacts appear in the archeology of Lower Mesopotamia during the early 4th millennium. Small amounts of natural gold have been found in Spanish caves used during the late Paleolithic period, c.
#AU PERIODIC TABLE FREE#
The earliest recorded metal employed by humans appears to be gold, which can be found free or " native".
#AU PERIODIC TABLE HOW TO#
Ancient people even figured out how to refine silver. The first evidence of silver mining dates back to 3000 B.C., in Turkey and Greece, according to the RSC. History Īll the elements of the group except roentgenium have been known since prehistoric times, as all of them occur in metallic form in nature and no extraction metallurgy is necessary to produce them.Ĭopper was known and used around 4000 BC and many items, weapons and materials were made and used with copper. Copper, silver, and gold all occur naturally in elemental form. They were most likely the first three elements discovered. Group 11 is also known as the coinage metals, due to their usage in minting coins -while the rise in metal prices mean that silver and gold are no longer used for circulating currency, remaining in use for bullion, copper remains a common metal in coins to date, either in the form of copper clad coinage or as part of the cupronickel alloy.
Thank you for being concerned with us and I wish you for your continued success.Group 11, by modern IUPAC numbering, is a group of chemical elements in the periodic table, consisting of copper (Cu), silver (Ag), and gold (Au), and roentgenium (Rg), although no chemical experiments have yet been carried out to confirm that roentgenium behaves like the heavier homologue to gold. Show Electronic configuration of Gold.ĭear Student, I appreciate your efforts and hard work that you all had put in.
Position: Gold is place in periodic table at 6 th period ( row) and 11 th column ( group)Ĭolour : Gold show slightly reddish yellow colourīoiling point : Boiling point of gold is 2836 0 C In gold 79 proton and 118 neutron is present In gold 79 proton and 79 Electron is presentĪtomic weight/ mass: Atomic weight of Gold is 196.97
And symbol is derived from Latin ‘aurum gold ‘ Physical properties :Ītomic symbol: Atomic symbol of gold is AuĪtomic number : Atomic number of gold is 79 The name is derived from Anglo – sexon for the metal. It is easily found in nature.ĭiscovery Good is discovered from 3000 B. Define: Gold is inner transition metal place below silver.


 0 kommentar(er)
0 kommentar(er)
